|
Ink Dep.
|
|
|
|
|
Definition
Ink
is a liquid that
contains pigments;
Itís used to color
a surface to produce an image, text, or design. Ink is used for drawing, writing with a pen, brush, or quill. Thicker inks, in
paste form, are used extensively in letterpress
and lithographic
printing.
Ink can be a
complex medium, composed of solvents,
pigments, dyes, resins,
lubricants,
solubilizers,
surfactants,
particulate matter, fluoresces,
and other materials. The components of inks serve many
purposes; the inkís carrier, colorants, and other additives
control flow and thickness of the ink and its appearance when dry.
Pigment inks are
used more frequently than dyes because they are more
color-fast, but they are also more expensive, less consistent
in color, and have less of a color range than dyes
A pigment
is a material that changes the color of reflected or
transmitted light
as the result of wavelength-selective absorption. This physical process differs
from fluorescence,
phosphorescence,
and other forms of luminescence, in which a material emits light.
Many materials
selectively absorb certain wavelengths
of light. Materials that humans have chosen and developed for
use as pigments usually have special properties that make them
ideal for coloring other materials. A pigment must have a high
tinting
strength relative to the materials it colors. It must be
stable in solid form at ambient temperatures.
For industrial
applications, as well as in the arts, permanence and stability
are desirable properties. Pigments that are not permanent are
called fugitive. Fugitive pigments fade over time, or
with exposure to light, while some eventually blacken.
Pigments are used
for coloring paint, ink, plastic,
fabric,
cosmetics,
food and other materials.
Most pigments used in manufacturing and the visual
arts are dry colorants,
usually ground into a fine powder. This powder is added to a vehicle
(or binder), a relatively neutral or colorless material that suspends the pigment and gives the paint
its adhesion.
Inks
generally fall into four classes:
| *
Aq
ueous |
| *
Liquid |
| *
Paste |
| *
Powder |
A distinction is usually
made between a pigment, which is insoluble in the vehicle (resulting in a suspension), and a dye, which either is itself
a liquid
or is soluble in its vehicle (resulting in a solution). The
term biological pigment is used for all colored
substances independent of their solubility. A colorant
can be both a pigment and a dye depending on the vehicle it is
used in. In some cases, a pigment can be manufactured from a
dye by precipitating a soluble dye with a metallic
salt. The resulting pigment is called a lake
pigment. |
|

|
|
|
|
|
|
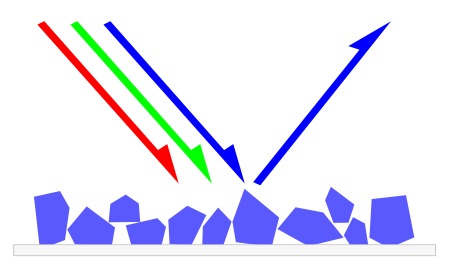
A wide variety of
wavelengths (colors) encounter a pigment. This pigment absorbs
red and green light, but reflects blue, creating the color
blue.
Pigments
appear the colors they are because they selectively reflect
and absorb certain wavelengths of visible
light.
White
light
is a roughly equal mixture of the entire spectrum of visible
light with a wavelength
in a range from about 380 or 400 nanometers
to about 760 or 780 nm. When this light encounters a pigment,
parts of the spectrum are absorbed
by the chemical
bonds
of conjugated
systems
and other components of the pigment. Some other wavelengths or
parts of the spectrum are reflected or scattered. Most
pigments are charge-transfer
complexes,
like transition
metal compounds,
with broad absorption
bands
that subtract most of the colors of the incident white light.
The new reflected light spectrum creates the appearance of a color.
Ultramarine
reflects blue light, and absorbs other colors. Pigments,
unlike fluorescent
or phosphorescent
substances, can only subtract
wavelengths from the source light, never add new ones.
So
a BLUE
PIGMENT is BLUE
because it doesn't reflect RED
and GREEN
light, or because it reflects all colors but the complementary
of the BLUE
one, which is ORANGE
A
PURPLE
PIGMENT is PURPLE
because it absorbs all GREEN
light.
The
appearance of pigments is intimately connected to the color of
the source light. Sunlight has a high color
temperature, and a fairly uniform spectrum, and is
considered a standard for white light. Artificial light
sources tend to have great peaks in some parts of their
spectrum, and deep valleys in others. Viewed under these
conditions, pigments will appear different colors.
Color
spaces used to represent colors numerically must specify their
light source. Lab
color measurements, unless otherwise noted, assume
that the measurement was taken under a D65 light source, or
"Daylight 6500 K", which is roughly the color
temperature of sunlight.
|
|

|
|
Sunlight
encounters Rosco R80 "Primary Blue" pigment. The
product of the source spectrum and the reflectance spectrum of
the pigment results in the final spectrum, and the appearance
of blue.
Other
properties of a color, such as its saturation or lightness,
may be determined by the other substances that accompany
pigments. Binders and fillers added to pure pigment chemicals
also have their own reflection and absorption patterns, which
can affect the final spectrum. Likewise, in pigment/binder
mixtures, individual rays of light may not encounter pigment
molecules, and may be reflected as is. These stray rays of
source light contribute to the saturation of the color. Pure
pigment allows very little white light to escape, producing a
highly saturated color. A small quantity of pigment mixed with
a lot of white binder, however, will appear desaturated and
pale, due to the high quantity of escaping white light.
|
|

Natural
ultramarine pigment in powdered form
|
|

Synthetic
ultramarine pigment is chemically identical to natural
ultramarine
|
|
|
 |













 |